Teeth Whitening Science
Many people are interested in whitening their teeth or getting into the teeth whitening business. Whereas not everyone needs to understand how the tooth appears more white at the molecular level, anyone wanting to offer teeth whitening services should be able to explain the science behind teeth whitening to their customers. If you are looking to whiten your teeth, it will help you to understand teeth whitening science so you can choose the solution that best helps you meet your goals.
What Is Teeth Whitening?
Teeth whitening, also known as bleaching, is the act of removing stains from the teeth, which in turn makes them look whiter. There are surface stains that reside on the tooth’s enamel. You can usually remove surface stains by simple mechanical means such as tooth brushing or polishing. Usually, the dentist polishes teeth. But even polishing only removes surface stains, and surface stains come back quite readily.
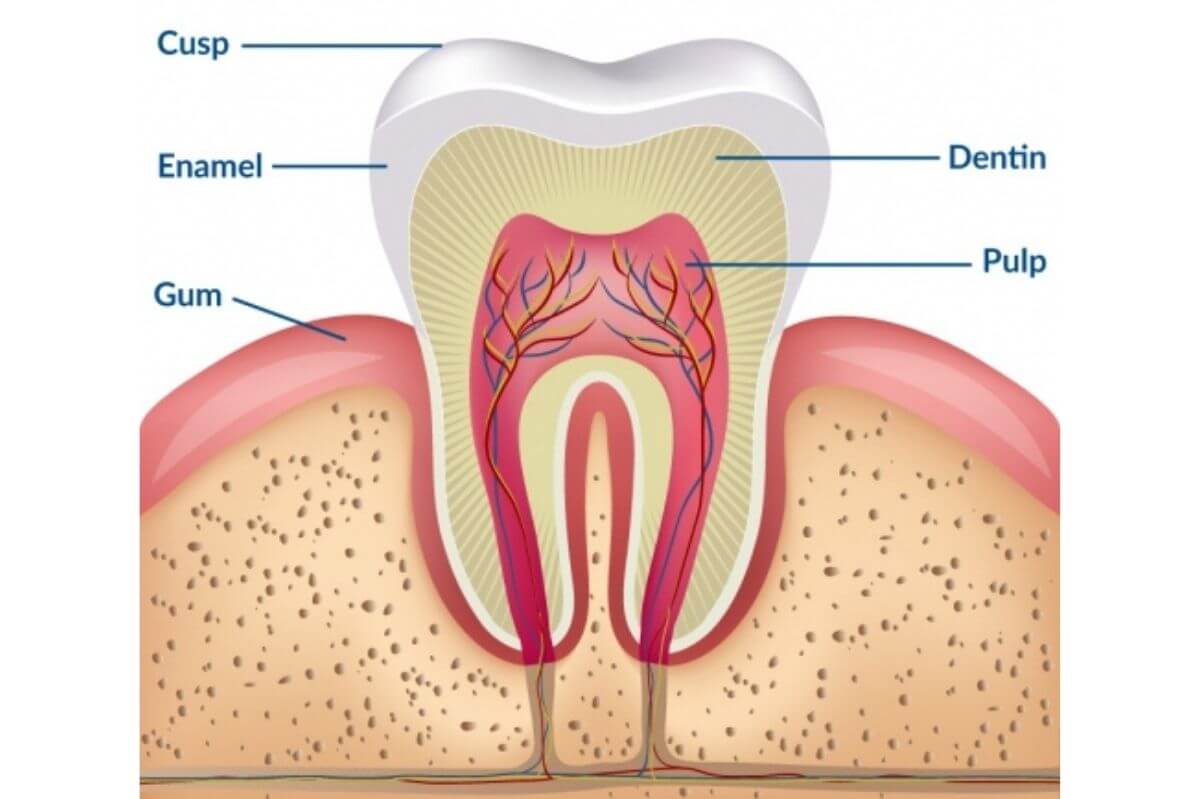
What we mean by teeth whitening is the bleaching or whitening of the stains on the dentin layer of the tooth. If you look at an anatomical drawing of a tooth, you’ll see that the outer layer of the teeth (the layer you feel when you touch your teeth), is the enamel. The enamel is a transparent layer, and it does not retain any stains in it, just on the surface of it.
Below the enamel is the dentin layer, which is the one that gives the tooth its color. It is here that your teeth get stained. The enamel layer is porous, so staining particles from coffee, tobacco, wine, tea, etc. can seep through these pores to reach the dentin, and that’s where they accumulate. The more of these particles get through, the more discolored (stained) your teeth become.
To whiten your teeth, you need to get a bleaching substance to the dentin layer. The best way to bleach anything is with oxygen radicals, but you can’t pump pure oxygen (or especially oxygen free radicals) to your teeth, much less through the enamel. The best-known teeth whitening chemical in the world, when we wrote this, is hydrogen peroxide. Hydrogen peroxide (H2O2) is NOT the same as oxygen (O2), but when hydrogen peroxide reacts, it breaks down into water (H2O) and oxygen radical (of which there are a few varieties). Oxygen radicals only live a fraction of a second, but for that moment, they desperately look for something to oxidize (or react with), and the stains on your teeth are just what the doctor ordered.
What the teeth whitening science and research shows is that when you apply hydrogen peroxide or carbamide peroxide gel to the teeth, the hydrogen peroxide breaks down on the tooth’s surface and travels through the pores in the enamel of the tooth until it reaches the dentin layer. There, the oxygen radicals react with the stained particles on the dentin and whiten them. They do NOT remove the staining molecules, but bleach them, removing their color. When you apply the right strength of hydrogen peroxide (or carbamide peroxide) with the correct frequency and duration to the teeth, they will appear significantly whiter.












